Introducing PoNS®
Introducing PoNS, a neuromodulation therapy for people with multiple sclerosis (MS)

Over 70% of people with MS develop trouble with their gait (ability to walk). This can have a big impact on simple day-to-day activities such as grocery shopping, walking to the mailbox, playing with the kids—even answering the phone on time.
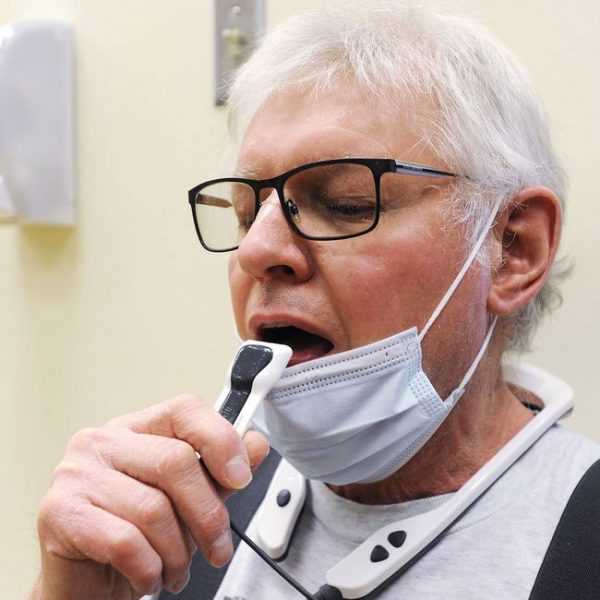
PoNS, short for Portable Neuromodulation Stimulator, is an orally applied therapy delivered by neurostimulation through a mouthpiece connected to a portable controller. It’s used, primarily at home, with physical rehabilitation exercise and improves walking in people with multiple sclerosis. Combined with physical rehabilitation exercise, PoNS can significantly improve your gait—to help keep you active and independent.
Watch how PoNS can help you
Get back in step with life
PoNS FAQs
Is PoNS right for me?

If you have MS and you are having trouble with your gait, PoNS combined with physical rehabilitation exercise can help. Take the assessment to find out if you should talk to your doctor about the benefits of starting therapy with PoNS.
How PoNS works

PoNS, short for Portable Neuromodulation Stimulator, is an innovative medical device. It includes a controller and mouthpiece that delivers mild stimulation to the surface of the tongue. This stimulation triggers a flow of neural impulses to the brain structures that control gait. PoNS is to be used in conjunction with physical rehabilitation exercise, referred to as PoNS Therapy®.
Use of PoNS while engaging in movement and coordination tasks promotes neuromodulation and neuroplasticity, activating brain pathways to improve gait.
What to expect with PoNS

In a clinical trial, individuals with mild to moderate MS and gait deficit who used PoNS along with PT for 14 weeks showed significant improvements in their gait compared to a sham device and PT.
In His Words
“After living with MS since 1999, gait difficulties took away my most valued treasure: quality adventures with my 13-year-old daughter. My focused PoNS therapy helped me improve my walking, and as a result of my improved walking I experienced freedoms such as increased walking speed, endurance, and distance. As a reward for her support and motivation, Rogue and I will vacation in New York City to walk the streets of Manhattan and enjoy our favorite Broadway musicals! Thank you for the experiences I thought were lost forever.”

Kerrie Walters
I have been living with MS for 35 years and have just completed my 14 week of PoNS Therapy. I am impressed with the results. The difference is apparent on before- and after- videos of me on the treadmill, and I feel steadier and more self-assured in my overall ability to move. I feel my improved gait since PoNS has led me to attempt things such as parking further from an entrance to a store or across the street from my destination instead of circling the block to find a closer spot. As a result of my improved walking, I was able to participate in a more active excursion on my vacation, such as walking, swimming, and scrambling through an underground cave in the Yucatan. My starting speed on the treadmill went from 1.3 to 2.6 during the protocol. PoNS Therapy was a serious commitment of time and energy but the improvements in my gait, at least for me, have changed my life.

Seeing Progress
“As someone who has had MS for over 40 years, I viewed PoNS® as something to not only expand my rehabilitation routine, but to push me both physically and mentally.”
“While I encountered barriers along the way and progress was slow and steady, my walking with assistance is much better when completing my exercises and daily activities.”

Modified Approach
“I will say that the PoNS® program was quite a vigorous workout for me, especially the first few weeks.”
“Propel Physiotherapy did a great job prescribing and modifying the exercises to suit my condition. My walking distance (with a rollator) improved substantially.”
Customized Care
“Though filled with hope when first learning about PoNS®, I had convinced myself to take the “wait and see” approach.”
“Then I found Novah Healthcare, with Clinic Director Dr. Trung Ngo and Resident Physiotherapist Asha Chaudhary. Their altruistic approach and flexibility to my availability were amazing. As a result of the customized program they put together with the PoNS® device, there was significant improvement in my gait.”

Carole Pirckle
“I was diagnosed with MS 8 years ago. After my diagnosis, I couldn’t do much, most of my days were spent on the couch. I knew I needed to do something to help me be able to walk. I learned about PoNS through an email from the MS Society and decided to give it a try.
Although the therapy was a commitment, and my 14-weeks occurred over the holidays, it gave me incentive to try harder. The exercises were easy to follow and complete at home. Thanks to PoNS® I am looking forward to walking to my daughter’s house which is close by.”

Helpful Resources
Find a Clinic
PoNS® is administered by physical therapists who have undergone specific training.
Find the clinic closest to you.
Questions to Ask Your Doctor
Ready to talk to your physician about PoNS?
Download our guide to talking to your doctor.

